About Us
Why should you use the terms “fine bubble” or “ultrafine bubble”, instead of “nanobubble”?
International Standard ISO-20480-1 Annexure A explains the reasons in detail. Below is an extract of the document that is self-explanatory.
“Although nano-objects are specified as 100 nm or less in ISO/TS 80004-1, there is no scientific or convincing evidence for the time being that there is a difference in physical phenomena of ultrafine bubbles with the dividing line of 100 nm.
Therefore, ISO/TC 281 does not use the term “nanobubble” throughout the fine bubble documents it develops, and instead uses “fine bubble” or “ultrafine bubble” in order to avoid confusion in the industry, the market, and in international standardization activities.”
MICRO and ULTRA-FINE BUBBLES (MB/UFB) ARE extremely small gas bubbles with several unique physical properties that make them VERY DIFFERENT from normal bubbles.
These properties make MB/UFB a SUPERIOR AERATION method for several worldwide applications.
Normal bubbles generated by a venturi, airstone, semi-flexible air diffusers, and alike produce bubbles too large (greater than 100µm) to promote the dissolution of the interior gas of the bubbles, rises quickly, and release the gas into the atmosphere.
Ultra-Oxygen solutions produce MB/UFB with a mean size of 15µm, thus smaller than 100µm, that promotes the dissolution of the interior gas of the bubbles, stays in the water due to their extremely low buoyancy, and releases the gas into the water in the most efficient way known to man.


Properties of Ultra-fine Bubbles
- Fine bubbles can be present in both liquids and solids.
- Fine bubbles can contain air or another gas. O2, O3, N, and CO2 also stay in the water and remain effective.
- The bubble can be held in place by surface tension or be surrounded by a coating, e.g. a lipid.
- Fine bubbles generated for various applications can vary in size, gas content, or bubble coating.
- The generation techniques used are also different.
- Very small; hence, some are not visible to the human eye or with a standard microscope.
- The image above represents the size and visibility of Micro and Ultrafine bubbles illuminated by a green laser using a macro lens and long camera exposure on a black background.
Why are Micro Bubbles and Ultrafine Bubbles so Amazing
Before jumping to the theory, we can achieve the results below with MB/UFB Technology CHEMICAL-FREE, which provides other excellent ENVIRONMENTAL FRIENDLY sustainable solutions that are EASY TO IMPLEMENT within 1 hour.

Ultrafine bubbles include bubbles of nano size.
- Ultrafine bubbles(UFB) are the smallest bubble size known to humanity, up to 10 000 times smaller than a human hair or about the size of a virus.
- Large interfacial area – At this scale, far more ultrafine bubbles can fit in the same water volume than other bubbles.
- They have several unique characteristics directly related to their miniature size, including neutral buoyancy, strong electric charge, and high transfer efficiency.
- Larger bubbles do not possess these characteristics, making them less beneficial in several applications than ultra-fine bubbles.
- Bioactivity application field – 10-40 µm (micrometre) size bubbles.
- Fluid physics application field – 100 µm (micrometre) size bubbles and less.
Slow rising velocity – Neutral buoyancy
The rising velocity of Micro Bubbles (MB) depends on the physical properties of liquids. Stokes equation tells us, for example, that a 10 µm MB rise only 20 cm in one hour. Knowing that microbubbles are tiny bubbles with a diameter of less than 100 µm and that the bubbles decrease in size and eventually disappear underwater because of the rapid dissolution of the interior gas, they lack enough buoyancy to reach the surface and instead follow Brownian motion. ISO published results after stopping to monitor the presence of UFB in a water body that it was still present in the water body after three months.
The net effect is that UFBs will remain suspended in water for months until they dissolve, travelling randomly throughout the body of water and efficiently aerating the entire water column. Subsequently, dissolved oxygen (DO) measured at the deepest parts of a water body will match the DO recorded near the surface. This unique behaviour enables MB and UFB to provide homogenous oxygen distribution throughout an entire body of water.
Most people ask, why not use a venturi, airstone, semi-flexible air diffusers, and the like? The answer is simple. The bubbles they generate rise too quickly.

Exponentially Larger Interfacial Surface Area
Equation A/V=6/d obtains the interfacial area of bubbles divided by volume A/V. With decreasing bubble diameter d, A/V increases and contributes to gas dissolution fraction.
Essentially this means that the smaller the bubble, the greater the surface area of the bubble exposed to the carrier liquid and a much greater gas transferability; when combined with the high internal gas pressure and lack of buoyancy, micro and ultrafine bubbles are amazingly efficient aeration mechanism.
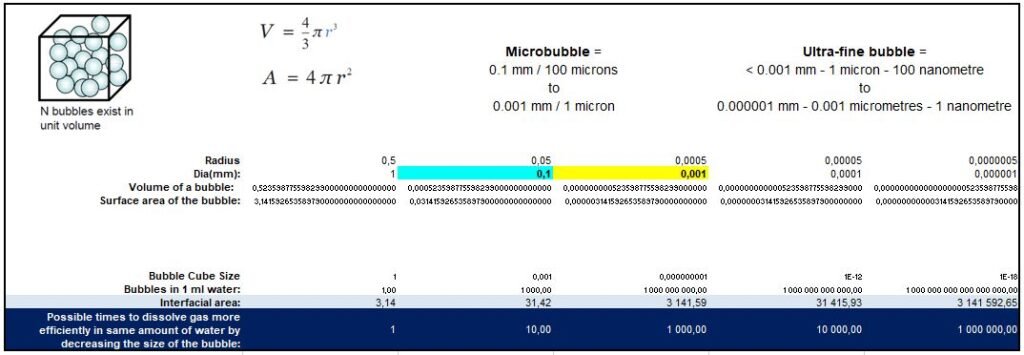
Large gas dissolution – Massive Gas Transfer Efficiency
The mass transfer rate from gas to a liquid, or dissolving rate, is measured in Mole Per Second (mol/s). Microbubbles(MB) and Ultrafine bubbles (UFB) unique properties ensure a massive increase in gas dissolution. When the mass transfer rate of the 1 mm bubble during the 1 mm rise is considered as 1, mass transfer rates of 10 µm and 100 nm bubbles become 108 and 1018, respectively, due to the decrease in rising velocity.
The larger surface area allows for increased mass transfer, ensuring virtually any gas is effectively delivered to water. The unique bubble act like gas batteries in solution; as the liquid dissolved gas is consumed, micro and ultrafine bubbles release the gas contained within into the surrounding liquid to replace the consumed dissolved gas and keep the solution in equilibrium according to Henry’s Law.
In short – Ultra-Oxygen Micro and Ultrafine generators produce bubble sizes that decrease due to the dissolution of the gas of the bubbles, thus increasing the internal gas pressure. Following Henry’s Law, the shrinking of the bubbles accelerates further the dissolution of gas and increases the internal gas pressure of the bubbles. Finally, most bubbles disappear in the water and not in the atmosphere.
Oxygen is consumed from the water by biology, chemistry, or off-gassing. Ultra-Oxygen MBs and UFBs rapidly diffuse more oxygen into the water, maintaining elevated dissolved oxygen levels. This additional gas reserve enables industries to utilise gases more cost-effectively than ever.
In a recent WWT trial done in a 10 000L tank and using an oxygen concentrator, Ultra-Oxygen (UO-400) generator achieved 37.5 mg/L oxygen in raw screened sewer water, thus over 400% saturation. Increasing the DO from 0,5mg/L to 21mg/L was possible within 3 hrs.
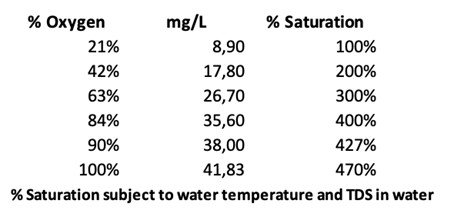
In nature, the gas dissolves into the water, depending on the gas pressure in the air just above the water. Aeration with Micro and Ultrafine bubbles creates high gas pressure conditions inside the bubbles floating in the water. Therefore, MB/UFB themselves can accelerate the dissolution of gas in the water without any chamber with high gas pressure. This technique has the significant advantage to realise quick dissolution of the gas into the water in open space at VERY LOW COSTS.
Micro and Ultrafine bubbles’ high gas dissolution rate decreases the staggering energy demand to aerate water in wastewater treatment facilities, as well as other agriculture, aquaculture, commercial, and many other applications that require aeration or a good supply of oxygen to saturate the water body.
Microbubble and ultrafine bubble longevity and large interfacial surface area are proven to be the most efficient aeration technique in the world. If you are not using MB and UFB technology for aeration in your process, you may be paying up to 200% more for your energy bill than needed.
Contact us today to help you determine where and how much you can save on energy costs.
With ever-rising energy costs, Ultra-Oxygen Micro and Ultrafine bubble technology will allow plant operators to drastically decrease their energy costs, thus having additional money available to spend on much-needed technology upgrades worldwide.
At what size do micro bubbles start to contract, and why is this important?
Scientific results published by Ohnari in 2007 using a swirling-type MB generator revealed that the pressure at gas suction becomes 0.06 MPa less than the atmospheric pressure. Microbubbles (MBs) were formed under the combined force of atmospheric pressure and static pressure. The bubbles were pressurised by liquid and began to contract, and dissolution occurred.
On the other hand, bubbles larger than 65 μm (microns) expanded. Beneficial Micro bubbles smaller than 65 μm (microns) contract, bubbles under 15 μm contracted more rapidly and formed Ultrafine bubbles when reaching the size of 1 micron (1000 nanometre) and smaller.
Why EXPENSIVE “NANOBUBBLE” equipment is overrated
Ultra-Oxygens micro and ultrafine bubble generators were used in many case studies and scientific publications that prove that all you need is Micro bubbles smaller than 65 microns that contract to Ultrafine bubbles to benefit from using UO2-MB/UFB technology to its full potential.
Only addressing bubble size without considering the oxygen demand over time is fruitless.
Determining your UO2-Factor is one of the most critical Value Engineering exercises you should do before investing in MB/UFB technology or starting to design your site-specific solution for your project.
- UO2-FACTOR = UFB $ / max DO in mg/L
- Cost in USD of the MB/UFB generator, divided by the maximum dissolved oxygen level the generator can produce
- UO2-FACTOR = Less than 150 = Recommended
Micro and Ultrafine Bubbles Uniquely have a Negatively charged surface
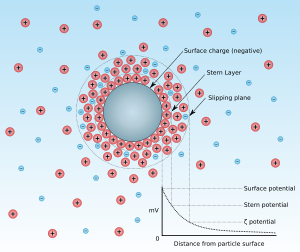
All bubbles naturally possess a surface charge, but the Micro bubble’s charge is negative between -30 and -40 mV independent of the bubble diameter. The smaller the bubble, the greater the negative surface charge.
Microbubbles smaller than 65-50 microns get compressed by ions at the gas-liquid interface.
This results in the ion concentration at the gas-liquid interface increasing and the internal pressure and temperature rising, leading to various phenomena.
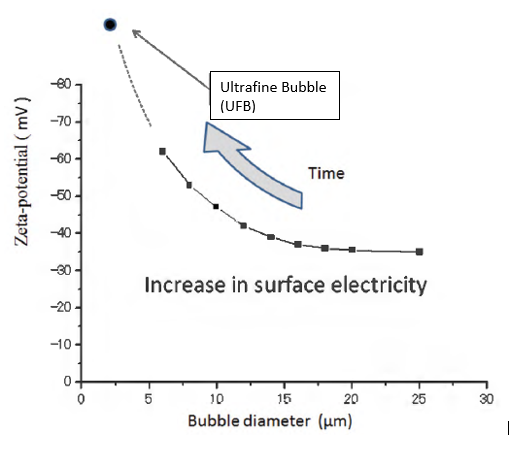
Micro and Ultrafine bubbles have a high zeta potential, the electrokinetic potential in colloidal dispersion. Through the testing done with zeta-sizers, it has been shown that the strong negative charge of Ultrafine bubbles (UFB) limits their coalescence, meaning the integrity of the bubble is preserved at any depth for extended periods of time. This means they won’t create larger bubbles and rise to the surface and release the gas within them, ensuring high bubble densities, long life, and high gas concentrations in solution, all ideal for aeration applications.
Furthermore, by increasing collision probability, micro and ultrafine bubbles’ high negative surface charge and high density significantly improve separation efficiency in flotation processes. By positively charging the surface of suspended materials and adsorbing it on the negatively charged surface of MB/UFB, we can apply it to the separation operations. Overall, this unique property is ideal for wastewater solids separation, oil, gas, and mining operators to greatly increase their flotation systems’ efficiency.
Zeta potential is a crucial indicator of the stability of colloidal dispersions. The higher the zeta potential, the more stable the behaviour of the colloidal particle. Ultrafine Bubbles (UFB) have a high zeta potential, meaning their interaction with surrounding colloidal particles is stable compared to larger bubbles. The higher zeta potential also causes excess ions to be formed and the creation of free radicals. Free radicals are atoms with an odd number of electrons that can be created when oxygen interacts with specific molecules. Free radicals are essential to processes such as combustion, atmospheric chemistry, polymerisation, plasma chemistry, biochemistry, and other chemical reactions.
Atoms that do not have an entire outer shell of electrons desperately seek out electrons wherever they can so they can become stable and inert. These unstable atoms, free radicals, Hydroxyl radical (HO), and Hydroxide [OH]− seek stability and try to steal an electron from whatever molecule happens to be nearby. The hydroxyl radical is often referred to as the “detergent” of the troposphere because it reacts with many pollutants, decomposing them through “cracking”, often acting as the first step to their removal. It also has a vital role in eliminating greenhouse gases like methane and ozone. Hydroxide functions as a base, a ligand, a nucleophile, and a catalyst. Hydroxide ion and hydroxy group are nucleophiles and can act as a catalyst in organic chemistry.
The surface charge is affected by the pH of water. An acidic 4.5pH will result in a 0mV and 8pH value of -100 mV.
Ultra-OXYGEN MB and UFB equipment allow us to provide our client with chemical free solutions that are welcomed and conducive to aerobic bacteria and kills off anaerobic bacteria.
Through the zeta potential observation during the collapsing microbubbles, it has been demonstrated that the ionic cloud around the bubble’s shrinking gas–water interface contributes to stabilising the residues by suppressing the gas dissolution to the surrounding water.

